Breast cancer is the most common cancer in women worldwide, but many immunotherapies have had limited success in treating aggressive forms of the disease.
“A deeper understanding of the immunobiology of breast cancer is critical to the success in harnessing immunotherapeutic approaches to improve breast cancer survival,” said Paula Bos, Ph.D., member of the Cancer Biology research program at VCU Massey Cancer Center and assistant professor in the Department of Pathology at the VCU School of Medicine.
New research findings from Bos, published in Cell Reports, identified a type of immune cells that acts as a major driver of breast cancer growth by preventing the accumulation of a specific protein that induces anti-tumor responses. This new knowledge could be utilized for the development of novel immunotherapeutic approaches to treat the disease.
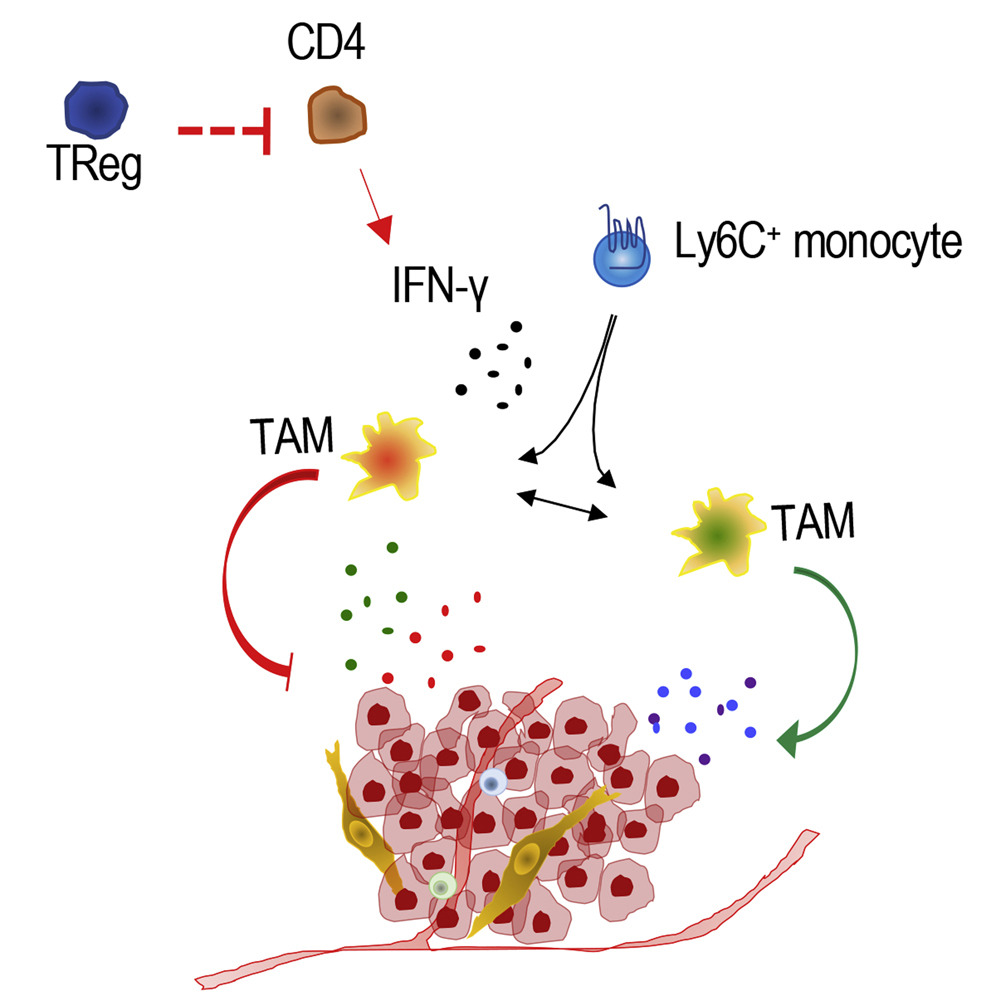
Regulatory T cells (Treg cells) are a special class of immune cells that possess a unique ability to suppress the function of other immune cells. This function serves to protect the organism from overreacting to certain molecules created within the body; however, in many cases it subdues the immune system’s ability to attack cancer cells. Therefore, Treg cells are often abundant in solid tumors, particularly breast cancers, and are commonly associated with worse outcomes.
In previous research, Bos demonstrated that targeting Treg cells in breast cancer models significantly reduced tumor growth and metastasis; however, it remained unclear on a molecular level why this tumor reduction was happening.
There is a specific protein called interferon gamma (IFN-g) that has powerful anti-tumor properties, including the activation of macrophages, which are cells that can initiate inflammation and prevent cancer growth.
Bos’ latest study suggests that Treg cells suppress IFN-g production by CD4 T lymphocytes (a type of white blood cells), further instigating disease progression. After analyzing breast cancer models in which Treg cells had been targeted and destroyed, Bos discovered an increased presence of IFN-g and functional reprogramming of macrophages into tumor-fighting cells.
“Additionally, we demonstrated better overall survival in human cancers with similar genetic patterns to those observed in mice with breast cancer whose Treg cells were eliminated,” Bos said.
This research is the first of its kind to study the mechanistic function of Treg cells in breast cancer.
Bos said these findings validate the potential for adoptive transfer therapeutics using macrophages programmed with the IFN-g protein to effectively treat breast cancer. Adoptive transfer refers to the process of transferring external cells into a patient to improve immune function or response.
“Our work raises the possibility that white blood cells can be extracted from cancer patients, reprogrammed outside of their body through brief exposure to the IFN-g protein and re-infused back into the patient, contributing to the generation of anti-tumor responses,” Bos said.
Bos is currently studying the function of Treg cells in metastatic cancer and plans to design follow-up studies testing the utilization of IFN-g as an adoptive transfer therapeutic agent in cancer mouse models.
Bos collaborated on this research with Mikhail Dozmorov, Ph.D., member of the Cancer Biology research program at Massey; Amy Olex, M.S., of the VCU C. Kenneth and Dianne Wright Center for Clinical and Translational Research; and Nicholas Clark, Ph.D., James deLigio, Comfort Effi, Leandro Martinez, and Steven Murdock of the VCU School of Medicine.
This study was supported by the VCU Department of Pathology; American Cancer Society Institutional Research Grant no. 14-192-40; METAvivor Research and Support; a Susan G. Komen Career Catalyst Grant; the National Center for Advancing Translational Sciences (UL1TR002649); and, in part, with funding from Massey’s NCI Cancer Center Support Grant (P30 CA016059).